Vapor Pressure Calculator Solvent Solution
Introduction:
In the realm of chemistry and physics, understanding vapor pressure is crucial, especially when dealing with solutions and solvents. Calculating the vapor pressure of a solution involves a meticulous process that requires precision. In this article, we will delve into the intricacies of vapor pressure calculations and provide you with a working calculator.
How to Use:
Using the vapor pressure calculator is straightforward. Enter the required parameters into the designated input fields, click the “Calculate” button, and instantly obtain the vapor pressure of the solution.
Formula:
The vapor pressure of a solution can be calculated using Raoult’s Law, which states that the vapor pressure of a solution is directly proportional to the mole fraction of each component in the solution. The formula is given by:
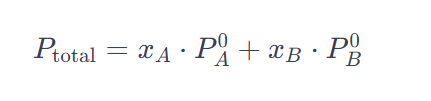
Where:

Example Solve:
Let’s consider a solution with two components, A and B, having mole fractions of 0.4 and 0.6, and vapor pressures of 20 and 30 mmHg, respectively.
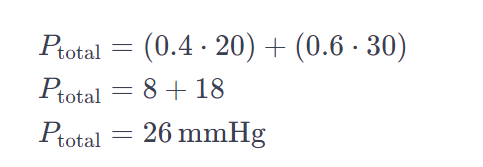
FAQs:
Q1: What units should be used for vapor pressure?
A1: Vapor pressure is commonly measured in millimeters of mercury (mmHg).
Q2: Can I input decimal values for mole fractions?
A2: Yes, the calculator allows decimal values for mole fractions.
Q3: Is Raoult’s Law applicable to all solutions?
A3: Raoult’s Law is an approximation that holds for ideal solutions with similar molecular interactions.
Conclusion:
Understanding vapor pressure is vital in various scientific applications. This calculator simplifies the computation process, ensuring accurate results for solutions and solvents. Utilize this tool to streamline your calculations in the world of chemistry and physics.
