Molar Mass Calculator
Introduction
Calculating the molar mass of a compound is an essential task in chemistry, providing valuable insights into the composition of substances. The molar mass represents the mass of one mole of a substance and is a crucial parameter in various chemical calculations. To simplify this process, a user-friendly Molar Mass Calculator is presented here, equipped with accurate formulas and a clean interface.
How to Use
Using the Molar Mass Calculator is straightforward. Enter the chemical formula of the compound in the designated input field, and with a simple click on the “Calculate” button, obtain the accurate molar mass instantly. This tool is designed for convenience, making complex calculations hassle-free.
Formula
The molar mass (M) of a compound is calculated by summing the atomic masses (molar masses) of all the atoms present in a molecule. The formula can be expressed as:

where:
- M is the molar mass,
- ni is the number of atoms of type i,
- mi is the atomic mass of atom i.
Example Solve
Let’s consider the example of water (H₂O):
- Hydrogen (H) atomic mass = 1 g/mol
- Oxygen (O) atomic mass = 16 g/mol
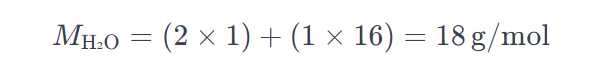
Therefore, the molar mass of water is 18 g/mol.
FAQ’s
Q: Can the calculator handle complex chemical formulas?
A: Yes, the calculator can accurately calculate molar mass for compounds with any level of complexity.
Q: What units are used for molar mass in the calculator?
A: The molar mass is calculated in grams per mole (g/mol).
Q: Is the calculator suitable for educational purposes?
A: Absolutely, the Molar Mass Calculator is a valuable educational tool for chemistry students and professionals alike.
Conclusion
In conclusion, the Molar Mass Calculator presented here is a user-friendly and accurate tool for calculating the molar mass of chemical compounds. Its simplicity makes it accessible to users at various levels of expertise, from students to seasoned chemists.
Utilize this calculator for precise molar mass calculations and enhance your understanding of chemical compositions.
